In a dark, dark room two scientists are sitting. One turns the black-black potentiometer, the second carefully looks into the dark-dark cathode tube. Fearfully? Actually, yes. Because what is happening is the real
In our time of continuous flows of information, the development of science and its popularization, with enthusiasm for social networks and various media platforms, the question of the quality of this information itself is more than ever. In addition to the viral spread, a real scourge of social networks becomes the instant finding and rallying of like-minded people around almost any idea - both radically politically tinged and completely absurd. If even supporters of ideas like a flat Earth are gaining a critical mass, so that their quantity and self-confidence allow them to maintain themselves and psychologically resist even the simplest and most logical arguments, what about more complex topics that require special knowledge? Of course, this concerns the mass consciousness. Specialists like these are hardly affected, because education allows them to distinguish facts from pseudoscience and media myths.
But much more insidious compared to pseudoscience is the case when a professional in the field of science for some reason fools himself. Either chasing a sensational discovery, or inspired by the results and unwilling to surrender, the scientist becomes an invisible harmful element within the scientific community. He is proud of his results, he publishes them, he provokes discussions. And even finds supporters for his discovery, which in fact is not. Phenomenon, which he invented unnoticed by himself, in the very process of his research, even without intent to forgery.
Origin of the term
The American chemist Irving Langmuir is known as a pioneer of
the adsorption isotherm equation and the winner of the 1932 Nobel Prize in Chemistry for his work in the field of surface phenomena. Even as a popularizer of science, he never published his research on the phenomenon of pathological science. More than thirty years after working in the laboratories of the General Electric company (and in the year of receiving the Nobel Prize he became its director), he, being in his old age, presented his views in a rather narrow circle of a specialized public - at a colloquium at Knolls Research Laboratory on December 18, 1953 .
 Irving Langmuir
Irving LangmuirThe term “pathological science” could have remained in the mere recollections of a few witnesses as a story about several scientific oddities. A report was recorded on an audiotape, subsequently lost. And only after the death of Langmuir, while analyzing his papers in the Library of Congress, was a long-playing record with a copy of that film found. This record, in turn, was transcribed by R.N. Hall and released by General Electric Laboratories catalog number No. 68-C-035 in April 1968. Subsequently, the transcript with the attached illustrative material was scanned, and now, after such a long journey, is available on the Internet.
Langmuir leads the analysis of cases that caused laughter in the audience even during his speech, just a couple of decades later, the experiments he described. But he draws the most serious conclusions from his observation - still, if the scientific community could only be so immature that it was fooled by just incorrectly conducting experiments - this is a reason to think seriously, to systematize such cases and prevent their occurrence.
The full text of the speech Langmuyur
in the original can be found here . If I want readers, I can translate the transcript entirely, a separate post. Here I will retell the essence of the examples described by him and the important conclusions made by him.
Davis Barnes Effect
In 1929, an interesting effect was discovered by a professor at Columbia University, USA, Bergen Davis. A brief idea of the experiment was as follows.
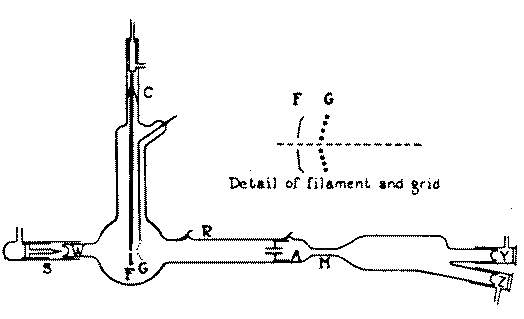 Installing Davis. A source
Installing Davis. A sourceThere is an alpha-active material (notorious polonium), from which a stream of alpha particles is obtained. They can be run through a vacuum tube (from point S in the figure). The flow of alpha particles flies strictly straight, but if you turn on the magnetic field nearby, then under its action the alpha particles
deviate by a known amount . Then, without a field, the rays will come to the end of the tube (Y), and when the field is applied, to the lateral process (Z).
Now parallel to the radioactive stream of particles we will launch a stream of electrons. Place the cathode (F) in the tube with a hole in the center. It will emit electrons, and radiation will go on through the hole. The idea is that two streams of particles are now parallel to each other: heavy alpha particles with a charge of 2+ and electrons with a charge of -. According to the idea of the researchers, the particles had to “recombine” (roughly speaking, merge), forming a stream of altered alpha particles with one positive charge instead of two. But the destination Z was calculated accurately, based on the speed of alpha particles and the magnitude of their charge. This means that "singly charged" alpha particles should have been deflected under the influence of a magnetic field weaker, without falling into the Y and Z ends of the tube. It remains only to insert a phosphorescent material into the tube at its Y- and Z-ends (Davis used zinc sulfide matrices), and you can manually count the flashes from each alpha particle arriving at the screen.
Let me remind you the idea: ordinary alpha particles under the influence of a magnetic field had to get into screen Z, and “singly charged” that absorbed an electron from the cathode would fly past. But Davis and his colleague Barnes made a startling discovery, from their point of view. To change the electron flow rate, they applied a different voltage to the cathode. And the energies on which they observed pronounced electron capture by alpha particles exactly coincided with the energies of the orbits
in the model of the Bohr atom ! Several such levels were discovered, in the range of the corresponding cathode voltages from 300 to 1000 volts. At the same time, each absorption peak lay in a very narrow region, of the order of 0.01 volts.
We now know that the Bohr model of the atom is incomplete and true only for the so-called hydrogen-like nuclei. But then the data of Davis and Barnes became a subject of discussion, moreover, the scientists themselves invited Langmuir to witness their experiment!
Langmuir responded to the offer, and with his colleague Dr. Whitney came to Davis to his laboratory at Columbia University, in New York. In a dark room, a colleague Davis Barnes demonstrated his experiments with the installation, counting the flashes on the phosphorescent screen in the dark. During the experiments Langmuir expressed his doubts to Barnes: first, at what level of the cathode glow does the effect begin to manifest, and does it depend on the electron flux density at all? Secondly, how is it that even with low electron fluxes of such a short joint flight is enough to recombine with alpha particles? And he received immediate answers: the effect does not depend on the flow of electrons, they will be captured even if the cathode is at room temperature. Anyway, according to Richardson's equation, electrons will be emitted by the cathode. Well, as for the small time of flight of particles in parallel, the electron is a wave, which means that it can theoretically exist anywhere in the tube and always finds with whom to recombine. Nevertheless, it was rather strange that in any conditions the recombination was always about 80%, regardless of the power of the electron flow.
Langmuir in detail describes all the shortcomings of the experiments performed. First of all, no one bothered to normalize the observed flashes of light for a time. Langmuir, with a stopwatch, spotted Barnes watching flashes from 70 to 110 seconds, claiming he always counted two minutes. And the very concept of flares was ambiguous - Langmuir noticed that not only “direct hits” of alpha particles, but also parasitic side flares beyond the visual field limit were visible to the microscope aimed at the zinc sulfide screen. Langmuir and Whitney ignored these lights, trying to count the flashes on their own, while Barnes seemed to take them into account in the experiment. Further, it was doubtful how Hull, the Barnes assistant, managed to set the exact required voltage. He turned the knob of a potentiometer, calibrated from 0 to 1000 V, and installed one hundredths of a volt there. In addition, at some point, Barnes did not like one of the experiments, where they did not find the peak they had previously found at 325.01 volts. 325.02 volts also did not give the desired result. Therefore, Hull set the value to 325.015 volts (!).
Watching him, Langmuir realized one thing. Although the whole thing was happening in a darkened room so that no stray light would interfere with counting flashes in the microscope, the scale of the potentiometer in front of Hull was lit. In the control series of experiments, the voltage was not applied, and Hull did not touch the potentiometer knob, simply leaning back in the chair. This could be seen by Barnes, which means that the
experiment was not blind in the most direct sense of the word. Next came Langhmyur. At first, he imperceptibly asked Halla to "move out" from the desired voltage for a tenth of a volt, then for a volt. Then, even in the control series, pretend that it regulates some kind of voltage with the handle of the device. As a result, when a series of measurements was typed, in which correct and erroneous data were divided equally (the
null hypothesis ), Langmuir stated to Barnes that he really did not measure anything. Not today, not before.
Barnes immediately replied that the vacuum tube was just gassed. And to the question, wasn’t it the installation where Davis obtained his data, he said: it’s true, but we’ve always carried out a test and control measurement, with and without voltage. Davis, in contrast to Barnes, did not give instant explanations, but was simply shocked and could not believe what was happening. Langmuir wrote a 22-page article reviewing the experiment of Davis and Barnes, and their experiments ceased to reproduce and quote.
Visible and invisible rays
The following example by Langmuir is in some way similar to the previous one. In 1903, the famous French scientist Prosper-Rene Blondlot, a member of the Academy of Sciences, experimented with x-ray sources.
According to him, if the x-ray source (heated platinum wire or Nernst lamp) is placed in an iron capsule, closed at one end with a thick layer of aluminum, then a stream of rays is obtained. He called them N-rays. A feature of their observation was that they manifested themselves in dimly lit objects. Blondlot argued that it was necessary to sit in the dark and look at a dimly lit object, such as a phosphorescent screen or a sheet of paper. In this case, in no case can not look at the source itself. Then, with proper training, it becomes possible to see the N-rays falling on the screen. Blondlot's research spread, he discovered the property of N-rays to stock up in materials, for example, saturate a brick with them, and then looked at the N-rays emitted by the brick. At the same time, he could not immediately bring a centner of bricks to the laboratory and study brighter N-rays, since their intensity remained unchanged and required a dark room and a “developed observation skill”.
In the case of Blondlot, an independent observer became interested in his experiments, R.U. Wood Wood was present at Blondlot’s new experiments, which decided to study in more detail the optical properties of their rays. Since aluminum was permeable to them, Blondlo went even further, making an aluminum prism (!) And beginning to scrupulously study the angles of refraction of N-rays. Wood, who was watching this, most unceremoniously refuted all the experiments of Blondlot: using the darkness that was so necessary in the laboratory, he simply hid the aluminum prism in his pocket.
The second case of pathological science described at the Langmuir colloquium with the radiant energy of very weak intensity belongs to Russia. Biologist Alexander Gurvich in the 1920s described biophotons - ultra-weak ultraviolet radiation emitted by plant roots. He described how the roots of onions, planted next to another, deviate to the first plant. In this case, the effect is not observed if there is a quartz plate between the plants, and ordinary glass that passes biophotons causes the described effect. Gurvich called these rays "mitogenetic," and, according to Langmuir, at that time there were many publications on this topic. It should be noted that in our time the existence of small doses of photons emitted by plants is not disputed. There are only discussions about their nature, as some kind of chemiluminescence, but not exactly about their ability to stimulate the growth and development of plants.
Another phenomenon that Langmuir paid attention to in his speech is the so-called Ellison effect. During his experiments in 1927, Fred Ellison discovered no more and no less than two new chemical elements called
alabamin and
virginia , as well as a number of isotopes. His research also provoked a stormy scientific debate, and, according to Langmuir, hundreds of scientific publications were devoted to the Ellison effect at the time.
Unlike imaginary rays or absolutely arbitrarily calculated bursts of light, Ellison’s installation was as complex as it is logical. It again used a flash of light, this time from an electric spark, and an external magnetic field. The light from the flash passed through a polarizer (the
prism of Nicolas ), and then through a solution of some substance placed in an electromagnetic coil. The magnetic field rotated the plane of polarized light in a liquid (
the Faraday effect ), and at the output it was possible to observe the intensity of light (coincidence or non-coincidence of the polarization plane). The idea was to excite the spark and the coil of the magnetic field from one source to measure the relaxation time in the solution — how long the rotation of the polarization plane is maintained. By introducing a compensating delay into the electrical circuit (a person who understands a car will have a vivid analogy with the ignition advance angle), it was possible to measure the relaxation time with surprising accuracy - up to 300 ps.
It turned out that many substances have their own characteristic time lag, moreover, complex compounds showed an additivity property. The signal from ethyl acetate was the sum of the signals from ethanol and acetic acid. The effect was consistently manifested from concentrations of 10 nmol and did not depend on further increasing the concentration, that is, there could be very few substances, but it was also well recorded. Ellison successfully detected existing compounds and discovered new elements and isotopes with his method. The Head of the Department of Chemistry, University of California, Wendell Latimer used the Ellison method and discovered the tritium isotope. According to Langmuir, he met with Latimer several years after his brief publication on tritium. He said that in a strange way, after this work, he was no longer able to repeat his own results using Ellison's method, although he was absolutely sure that he was doing, controlling and rechecking himself. At the same time, the American Chemical Society, after lively discussions, nevertheless refused to accept any more articles on this method for publication. An exception was made for one, only one work - but in it the authors handed over to Ellison himself two or three dozen solutions, encrypting the samples and strictly not disclosing their composition. He identified them all accurately, despite the micromolar concentration of some of them.
So what was it? Langmuir himself openly leaves this question to the listeners of his report, without discussing the nature of the origin of the effects that have clouded the mind to their pioneers. In addition to either working or unscientific effect of Ellison, he points out that in the case of Barnes and Davis there was no forgery, at the very beginning Barnes simply brought his observations to Davis, and after that he suddenly found them to coincide with the Bohr theory of the atom. But, despite the uncertainty in the very causes of the pathological science, Langmuir focuses on the characteristic features of the experiments, from which the main
Signs of Pathological Science
- The maximum observed effect is caused by a certain phenomenon of very low intensity, while the enhancement of its intensity does not give an increase in the effect. This turns out to be true for all the examples given. Davis and Barnes always recombined 80% of the alpha particles, Blondlot could not build an N-ray searchlight, simply irradiating a growing onion with an ultraviolet lamp did not give a “mitogenetic” effect, and Ellison didn’t care if one mole or one micromole in the flask relaxation of polarized light is not affected.
- The value of the effect is on the border of perception or requires numerous repetitions for statistical reliability. Both to achieve the required number of bulbous roots bent toward each other, and to obtain the required number of flashes from alpha particles, researchers conducted new and new experiments. Having chased a certain phantom, scientists no longer retreated until, by the absolute number, a sufficient number of evidence was gathered “in favor of” the observed effect.
- Extreme accuracy statements. Wood asked Blondlot how he measures the refraction of a beam 2 mm thick with hundredths of a millimeter in the same way as Langmuir laughed at Davis and Barnes, who controlled 0.01 volts with a kilovolt potentiometer knob. Phenomena of extremely low intensity require excessively accurate measurements of the effect, which, however, according to paragraph 1 does not vary in magnitude.
- Fantastic explanations that go against experiment. Blondlot argued that one should not expect behavior from the N-rays according to the laws of classical physics, since the principles by which they are distributed are clearly different. The source of N-rays were many objects - bricks, even people. And Barnes’s explanation about the wave properties of an electron, which makes it always in the right place of the vacuum tube and recombines, looks doubtful.
- All inconsistencies are resolved by different circumstances and explanations issued on the fly. Barnes had an immediate response to all Langmuir's remarks: the tube is gassed, electrons have wave properties, the cathode even emits electrons at room temperature, the matrix gives off radiation from radioactive contamination.
- The ratio of followers and critics at first is about 50/50, then the former gradually disappear. While critics do not break the theory completely, a new phenomenon is actively discussed in the scientific community and many works are published. But later, interest, publications and statements about success disappear from the followers, and a couple of decades later, even in the circle of specialists, Langmuir has to specifically clarify that there was a time when interest in some unusual method was very great.
And then what?
More than half a century has passed since that important, but few of whom were noticed by the Langmuir colloquium. Was his analysis useful? Does history still know examples of research that fall under the signs of pathological science? You can confidently say that yes.
In 1962, the Soviet chemist Nikolai Fedyakin, and after that, in separate experiments, a new form of water was discovered by the Corresponding Member of the USSR Academy of Sciences Boris Deryagin. As a result of long-term experiments with water in long thin capillaries, another phase arose in the aqueous medium, called watering. The properties of this water were impressive: the density increased, the boiling point sharply increased with a simultaneous drop in the freezing temperature. Unusual properties struck the imagination, and although the irrigation was not always possible to obtain, and capillaries with a diameter of 0.1 mm created additional difficulties in the experiments, she was taken seriously. Until the end of the 60s, however, this water remained behind the Iron Curtain due to the language barrier - articles about watering were published only in Russian-language peer-reviewed journals.
However, everything that penetrated because of the Iron Curtain was not in the best light thanks to the media mentioned at the beginning. In 1969, Ellis Lippinkot
published an article on the spectral properties of watering in Science , which leads to a flurry of publications in both peer-reviewed and mass publications. Some scientists repeat with success, and some cannot confirm the data of Deryagin, in the best traditions of an equal distribution of followers and skeptics according to Langmuir. In a society charged by the Cold War, opinions emerge about a “watering backlog” from the USSR, by analogy with a “
missile backlog ” in the strategic nuclear arsenal, and even parallels are drawn between the new water and the “ice-nine” from the famous Kurt Vonnegut novel “The Cradle for cats ”(the speech in the novel is about modified ice, which can irreversibly turn into itself all the water on Earth with which it contacts). , … ! , , General Electric. , , . , .
, . - . : , , . , : , ,
. 1973 .
Instead of an afterword
– . , , . , , . - .
, ,
EMDrive ?
..: , . .