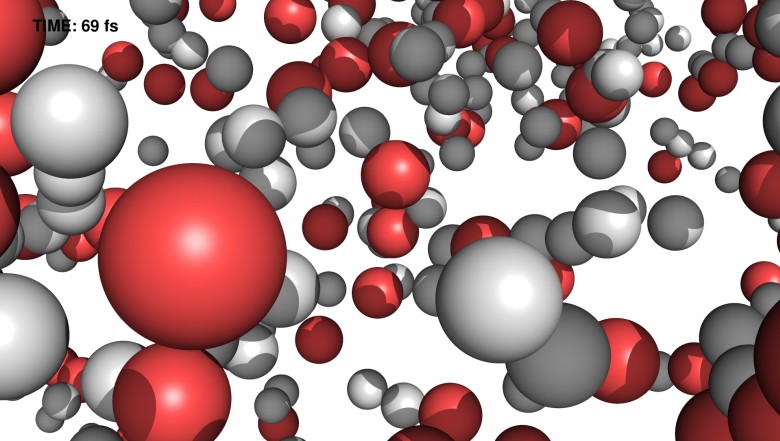
 After about 70 femtoseconds (quadrillionths of a second), most of the water molecules already decompose into hydrogen (white) and oxygen (red). Simulation: Carl Kalman, DESY / Uppsala University
After about 70 femtoseconds (quadrillionths of a second), most of the water molecules already decompose into hydrogen (white) and oxygen (red). Simulation: Carl Kalman, DESY / Uppsala UniversityTo study the exotic properties of matter in extreme conditions, scientists from the German research center for particle physics DESY and Uppsala University (Sweden)
conducted an experiment on ultrafast heating of water with an x-ray laser (razer) - and looked at whether the result coincides with the simulation.
Usually, heating while boiling water consists in transmitting kinetic energy to molecules through vibration by means of convection or heat radiation. But in this case, physicists used a different method, where energy is transmitted through ionization by single femtosecond pulses of a free electron X-ray laser. This causes a rapid ionization with the appearance of an exotic state of plasma, known as
warm dense matter (WOM).
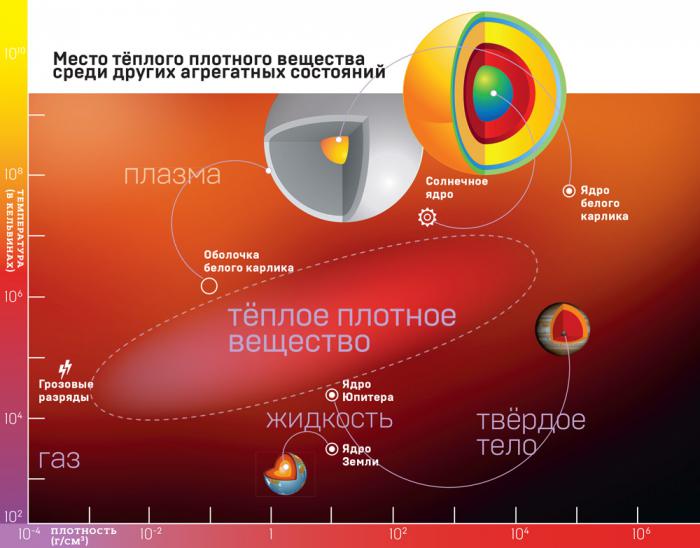
A warm dense substance (TPR) is an aggregate state of a substance, which by its parameters lies between a solid body and an ideal plasma. It is too dense to be described as plasma, and too hot to relate to condensed matter physics. In other words, it is a cross between a plasma and a solid body. It is much denser than plasma (from 0.01 to 100 g per cm³), and in some cases it has a specific weight of two times more than the solid substance from which it was obtained. In general, a kind of
substance Schrödinger .
The current experiment to get TPV from water was carried out by a group of scientists led by Carl Kalman (Carl Caleman) from the Center for the Study of Free Electron Lasers (CFEL) at DESY. The heating of the molecules with simultaneous state studies was carried out using a X-ray free electron laser at the SLAC National Accelerator Laboratory (USA). Razer carried out extremely intense ultrashort 6.86 keV X-ray flashes (more than 10
6 J / cm²) in a jet of water.

“This is clearly not the usual way to boil water,”
says Kaleman. “Usually, when heated, molecules simply shake themselves stronger and stronger. Our heating is fundamentally different. Energy X-rays knock electrons from water molecules, thereby destroying the balance of electrical charges. Suddenly, the atoms experience a strong repulsive force and begin to move rapidly. ”
In less than 75 femtoseconds, water undergoes a phase transition from liquid to plasma. Plasma is a state of matter in which electrons are removed from atoms, resulting in a kind of electrically charged gas.
“But during the transformation of a liquid into a plasma, water still maintains the density of the liquid, since the atoms have not yet had time to move significantly,” explains the co-author of the experiment, Olof Jönsson of Uppsala University. Such an exotic state of matter cannot be found in the natural state on Earth: “It has the same characteristics as some plasmas on the Sun and in the gas giant Jupiter, but only lower density. Meanwhile, it is hotter than the core of the Earth. "
Conducting an experiment on the water allows you to better know the properties of water in such an exotic state. This is all the more important given some of the truly unique properties of this substance: “Water is really a strange liquid, and if not for its features, then many things on Earth would not be as they are, especially life,” Jonsson emphasized. Water has many anomalous characteristics and properties, including density, heat capacity and thermal conductivity.
DESY plans to study water anomalies more closely in the framework of the projects of the future Center for Water Science, which is planned to be opened at DESY.
The experiment helped to develop methods for tracking single molecules using x-ray lasers. Scientists say that X-ray free-electron lasers "open the door to a new era of structural biology, allowing you to take biomolecules and track the dynamics that are inaccessible using existing methods."
The scientific article was
published on May 14, 2018 in the journal
PNAS (doi: 10.1073 / pnas.1711220115,
pdf ).